O Number Of Valence Electrons

Four covalent bonds. Carbon has four valence electrons and here a valence of four. Each hydrogen atom has one valence electron and is univalent.
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemic bond if the outer beat is not airtight. In a unmarried covalent bond, both atoms in the bail contribute one valence electron in order to class a shared pair.
The presence of valence electrons tin determine the element's chemical properties, such as its valence—whether it may bond with other elements and, if then, how readily and with how many. In this way, a given element's reactivity is highly dependent upon its electronic configuration. For a main-group element, a valence electron can exist only in the outermost electron shell; for a transition metal, a valence electron can also be in an inner shell.
An atom with a closed trounce of valence electrons (corresponding to a noble gas configuration) tends to be chemically inert. Atoms with ane or two valence electrons more than a closed shell are highly reactive due to the relatively depression free energy to remove the extra valence electrons to grade a positive ion. An atom with one or two electrons fewer than a closed shell is reactive due to its tendency either to proceeds the missing valence electrons and form a negative ion, or else to share valence electrons and class a covalent bond.
Similar to a core electron, a valence electron has the ability to absorb or release energy in the class of a photon. An energy gain tin can trigger the electron to move (jump) to an outer beat out; this is known as atomic excitation. Or the electron can fifty-fifty intermission gratis from its associated atom's shell; this is ionization to class a positive ion. When an electron loses energy (thereby causing a photon to be emitted), then it can movement to an inner vanquish which is not fully occupied.
Overview [edit]
Electron configuration [edit]
The electrons that make up one's mind valence – how an atom reacts chemically – are those with the highest energy.
For a main-group element, the valence electrons are defined as those electrons residing in the electronic shell of highest primary breakthrough number n.[i] Thus, the number of valence electrons that it may take depends on the electron configuration in a simple way. For case, the electronic configuration of phosphorus (P) is 1s2 2s2 2p6 3stwo 3p3 so that there are 5 valence electrons (3sii 3p3), corresponding to a maximum valence for P of five as in the molecule PF5; this configuration is normally abbreviated to [Ne] 3sii 3p3, where [Ne] signifies the core electrons whose configuration is identical to that of the element of group 0 neon.
Nevertheless, transition elements accept partially filled (n−1)d energy levels, that are very close in free energy to the northward s level.[2] Then equally opposed to main-group elements, a valence electron for a transition element is divers every bit an electron that resides outside a noble-gas core.[3] Thus, by and large, the d electrons in transition metals behave equally valence electrons although they are not in the outermost shell. For instance, manganese (Mn) has configuration 1sii 2s2 2p6 3s2 3p6 4s2 3d5; this is abbreviated to [Ar] 4s2 3d5, where [Ar] denotes a cadre configuration identical to that of the noble gas argon. In this atom, a 3d electron has energy similar to that of a 4s electron, and much higher than that of a 3s or 3p electron. In upshot, at that place are possibly 7 valence electrons (4sii 3dv) outside the argon-similar core; this is consequent with the chemic fact that manganese can have an oxidation state as high equally +7 (in the permanganate ion: MnO −
4 ).
The farther right in each transition metal serial, the lower the energy of an electron in a d subshell and the less such an electron has valence backdrop. Thus, although a nickel atom has, in principle, x valence electrons (4s2 3d8), its oxidation state never exceeds four. For zinc, the 3d subshell is consummate in all known compounds, although it does contribute to the valence ring in some compounds.[four]
The d electron count is an alternative tool for understanding the chemistry of a transition metal.
The number of valence electrons [edit]
The number of valence electrons of an chemical element tin can be determined by the periodic table group (vertical column) in which the chemical element is categorized. With the exception of groups 3–12 (the transition metals), the units digit of the group number identifies how many valence electrons are associated with a neutral cantlet of an element listed under that particular column.
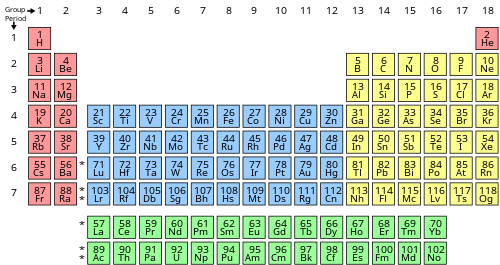
| Periodic table block | Periodic table group | Valence electrons |
|---|---|---|
| southward | Grouping ane (I) (alkali metals) | ane |
| Group 2 (II) (alkaline earth metals) and helium | two | |
| f | Lanthanides and actinides | iii–16[a] |
| d | Groups 3-12 (transition metals) | 3–12[b] |
| p | Group 13 (III) (boron group) | 3 |
| Group fourteen (Iv) (carbon group) | 4 | |
| Group fifteen (V) (pnictogens or nitrogen group) | 5 | |
| Group 16 (VI) (chalcogens or oxygen group) | vi | |
| Group 17 (Seven) (halogens) | 7 | |
| Group 18 (VIII or 0) (noble gases) except helium | 8 |
- ^ Consists of northdue south, (n−2)f, and (n−1)d electrons.
- ^ Consists of due norths, and (n−one)d electrons.
Helium is an exception: despite having a 1s2 configuration with 2 valence electrons, and thus having some similarities with the alkaline metal earth metals with their northdue south2 valence configurations, its shell is completely full and hence it is chemically very inert and is usually placed in group eighteen with the other noble gases.
Valence shell [edit]
The valence shell is the gear up of orbitals which are energetically attainable for accepting electrons to form chemical bonds.
For main-group elements, the valence crush consists of the ns and northwardp orbitals in the outermost electron beat out. For transition metals the orbitals of the incomplete (n−1)d subshell are included, and for lanthanides and actinides incomplete (northward−two)f and (northward−1)d subshells. The orbitals involved can be in an inner electron shell and do not all correspond to the same electron shell or principal breakthrough number n in a given element, but they are all at similar distances from the nucleus.
| Element type | Hydrogen and helium | p-cake (main-group elements) | d-block (Transition metals) | f-block (Lanthanides and actinides) |
|---|---|---|---|---|
| Valence orbitals[5] |
|
|
|
|
| Electron counting rules | Duet/Duplet rule | Octet rule | eighteen-electron rule | 32-electron dominion |
Equally a full general rule, a main-group element (except hydrogen or helium) tends to react to course a s2p6 electron configuration. This tendency is called the octet rule, because each bonded atom has viii valence electrons including shared electrons. Similarly, a transition metal tends to react to form a d10siipvi electron configuration. This tendency is called the 18-electron dominion, because each bonded atom has 18 valence electrons including shared electrons.
Chemical reactions [edit]
The number of valence electrons in an atom governs its bonding behavior. Therefore, elements whose atoms can have the aforementioned number of valence electrons are grouped together in the periodic tabular array of the elements.
The most reactive kind of metallic element is an brine metal of group 1 (due east.k., sodium or potassium); this is because such an atom has simply a single valence electron. During the formation of an ionic bond, which provides the necessary ionization energy, this one valence electron is easily lost to grade a positive ion (cation) with a closed shell (eastward.one thousand., Na+ or K+). An element of group i earth metal of group 2 (east.m., magnesium) is somewhat less reactive, because each atom must lose ii valence electrons to form a positive ion with a airtight shell (e.g., Mg2+).
Within each group (each periodic table column) of metals, reactivity increases with each lower row of the table (from a calorie-free chemical element to a heavier chemical element), because a heavier element has more electron shells than a lighter element; a heavier element's valence electrons exist at higher principal quantum numbers (they are further away from the nucleus of the cantlet, and are thus at higher potential energies, which means they are less tightly spring).
A nonmetal atom tends to concenter boosted valence electrons to attain a full valence shell; this tin exist accomplished in one of two ways: An atom can either share electrons with a neighboring cantlet (a covalent bond), or it can remove electrons from another cantlet (an ionic bond). The most reactive kind of nonmetal chemical element is a halogen (eastward.m., fluorine (F) or chlorine (Cl)). Such an cantlet has the post-obit electron configuration: siipfive; this requires only 1 boosted valence electron to form a closed shell. To grade an ionic bond, a element of group vii atom tin can remove an electron from another atom in order to form an anion (e.g., F−, Cl−, etc.). To form a covalent bond, i electron from the halogen and one electron from some other cantlet form a shared pair (due east.1000., in the molecule H–F, the line represents a shared pair of valence electrons, 1 from H and 1 from F).
Within each group of nonmetals, reactivity decreases with each lower row of the table (from a light element to a heavy element) in the periodic table, considering the valence electrons are at progressively college energies and thus progressively less tightly bound. In fact, oxygen (the lightest element in grouping 16) is the most reactive nonmetal after fluorine, even though it is not a halogen, because the valence beat of a halogen is at a higher main quantum number.
In these unproblematic cases where the octet rule is obeyed, the valence of an cantlet equals the number of electrons gained, lost, or shared in order to form the stable octet. Notwithstanding, there are likewise many molecules that are exceptions, and for which the valence is less conspicuously defined.
Electric conductivity [edit]
Valence electrons are also responsible for the electrical conductivity of an chemical element; equally a result, an element may exist classified as a metal, a nonmetal, or a semiconductor[ clarification needed ] (or metalloid).[ commendation needed ]
| one | 2 | three | four | 5 | six | seven | 8 | 9 | ten | 11 | 12 | thirteen | 14 | 15 | sixteen | 17 | 18 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grouping → | ||||||||||||||||||||||||||||||||
| ↓ Menstruum | ||||||||||||||||||||||||||||||||
| 1 | H | He | ||||||||||||||||||||||||||||||
| two | Li | Exist | B | C | N | O | F | Ne | ||||||||||||||||||||||||
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||
| 4 | K | Ca | Sc | Ti | V | Cr | Mn | Iron | Co | Ni | Cu | Zn | Ga | Ge | Equally | Se | Br | Kr | ||||||||||||||
| 5 | Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||
| half-dozen | Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | West | Re | Os | Ir | Pt | Au | Hg | Tl | Lead | Bi | Po | At | Rn |
| vii | Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Dr. | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| | ||||||||||||||||||||||||||||||||
| Metal Metalloid Nonmetal Unknown backdrop Background color shows metallic–metalloid–nonmetal tendency in the periodic table | ||||||||||||||||||||||||||||||||
Metallic elements by and large have high electrical conductivity when in the solid state. In each row of the periodic table, the metals occur to the left of the nonmetals, and thus a metallic has fewer possible valence electrons than a nonmetal. However, a valence electron of a metal atom has a pocket-sized ionization energy, and in the solid-state this valence electron is relatively free to get out one atom in order to associate with another nearby. Such a "free" electron can be moved under the influence of an electric field, and its motility constitutes an electric current; it is responsible for the electrical conductivity of the metallic. Copper, aluminium, silvery, and gold are examples of practiced conductors.
A nonmetallic element has low electrical conductivity; information technology acts as an insulator. Such an chemical element is constitute toward the right of the periodic table, and it has a valence shell that is at least half full (the exception is boron). Its ionization free energy is large; an electron cannot leave an atom easily when an electric field is applied, and thus such an element can behave only very pocket-sized electrical currents. Examples of solid elemental insulators are diamond (an allotrope of carbon) and sulfur.
A solid chemical compound containing metals tin also be an insulator if the valence electrons of the metal atoms are used to form ionic bonds. For example, although elemental sodium is a metal, solid sodium chloride is an insulator, because the valence electron of sodium is transferred to chlorine to form an ionic bail, and thus that electron cannot be moved hands.
A semiconductor has an electrical conductivity that is intermediate between that of a metallic and that of a nonmetal; a semiconductor as well differs from a metallic in that a semiconductor'south conductivity increases with temperature. The typical elemental semiconductors are silicon and germanium, each atom of which has four valence electrons. The properties of semiconductors are all-time explained using band theory, as a effect of a small energy gap between a valence band (which contains the valence electrons at absolute zip) and a conduction band (to which valence electrons are excited by thermal energy).
References [edit]
- ^ Petrucci, Ralph H.; Harwood, William S.; Herring, F. Geoffrey (2002). General chemical science: principles and modern applications (eighth ed.). Upper Saddle River, Northward.J: Prentice Hall. p. 339. ISBN978-0-13-014329-7. LCCN 2001032331. OCLC 46872308.
- ^ THE ORDER OF FILLING 3d AND 4s ORBITALS. chemguide.co.u.k.
- ^ Miessler G.Fifty. and Tarr, D.A., Inorganic Chemistry (2nd edn. Prentice-Hall 1999). p.48.
- ^ Tossell, J. A. (one November 1977). "Theoretical studies of valence orbital binding energies in solid zinc sulfide, zinc oxide, and zinc fluoride". Inorganic Chemical science. 16 (11): 2944–2949. doi:10.1021/ic50177a056.
- ^ Chi, Chaoxian; Pan, Sudip; Jin, Jiaye; Meng, Luyan; Luo, Mingbiao; Zhao, Lili; Zhou, Mingfei; Frenking, Gernot (2019). "Octacarbonyl Ion Complexes of Actinides [An(CO)8]+/− (An=Thursday, U) and the Role of f Orbitals in Metal–Ligand Bonding". Chem. Eur. J. 25 (l): 11772–11784. doi:ten.1002/chem.201902625. PMC6772027. PMID 31276242.
External links [edit]
- Francis, Eden. Valence Electrons.
O Number Of Valence Electrons,
Source: https://en.wikipedia.org/wiki/Valence_electron
Posted by: emersonwaallovar.blogspot.com


0 Response to "O Number Of Valence Electrons"
Post a Comment